Rigorous Sample Size Justification for a Long-Acting HIV Therapy Trial
Determining the appropriate sample size is a cornerstone of any clinical trial, ensuring both scientific validity and ethical resource allocation. In our recent study evaluating a novel long-acting HIV therapy, we employed a meticulous approach to sample size calculations, driven by the need to demonstrate non-inferiority for key outcomes and gather robust safety data. This article details the rationale behind our chosen sample sizes for the primary,secondary,and substudy analyses.
Primary Outcome: Demonstrating Non-Inferiority
for the primary endpoint,we aimed to demonstrate non-inferiority with a 10% margin,using a 5% two-sided meaning level and achieving 90% power. These parameters led us to a required sample size of 238 participants - 119 in each treatment arm. Importantly, the power to detect non-inferiority for this primary outcome, given our chosen parameters, was exceptionally high, approaching 100%.
Key Secondary Outcome: Accounting for Hierarchical Testing
The key secondary outcome, virological failure, required a more conservative approach. Based on data from previous 8-weekly long-acting trials33, we anticipated a failure rate of 1.7% in both treatment groups.
* We utilized a hierarchical testing procedure to control for multiple comparisons.
* This necessitated a 4% non-inferiority margin, a 5% two-sided significance level, and 85% power.
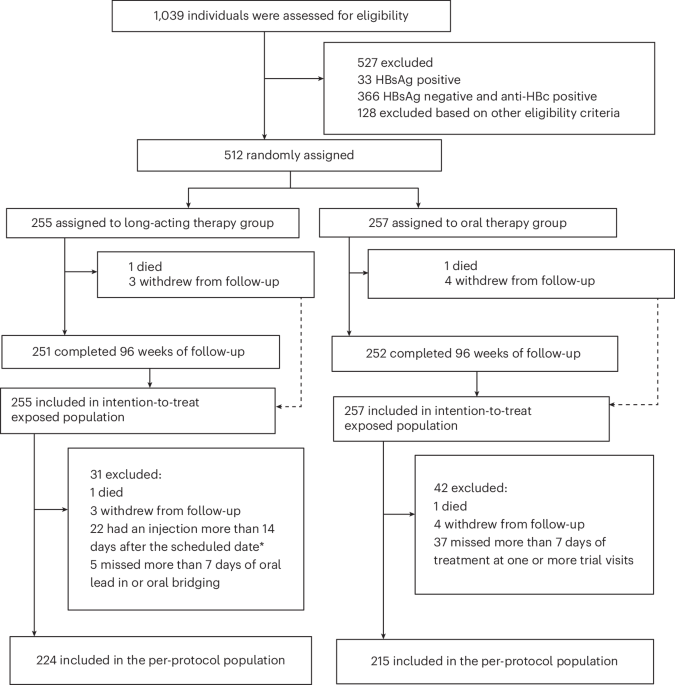
* these criteria indicated a need for 512 participants (256 per group).
To ensure adequate power for both the primary and secondary analyses, and to bolster the safety data available for broader adoption of long-acting therapy, we opted to proceed with the larger sample size of 512 participants. This decision prioritized thorough data collection and maximized confidence in our findings.
DEXA Substudy: Assessing Body Composition Changes
A dedicated substudy focused on changes in body composition, specifically trunk fat mass, using Dual-energy X-ray absorptiometry (DEXA) scans.
* We targeted a minimum of 120 participants across four study sites.
* This sample size was calculated to provide at least 90% power to detect a clinically meaningful difference of 1.2 kg between treatment arms.
* The calculation was based on a standard deviation of 2 kg, informed by body composition data from treatment-naive individuals initiating HIV treatment in Africa2, and a 5% alpha level.
Recognizing the inherent uncertainties in sample size estimations – particularly regarding attrition and the potential for exploring additional body composition outcomes - we allowed sites to enroll beyond the minimum target.This versatility ensured a robust dataset for comprehensive analysis.
Analytical Approach & Transparency
All statistical analyses were performed using Stata version 16.1 (statacorp),with the exception of efficacy analyses involving proportion differences,wich were conducted in R version 4.3.3. A detailed statistical analysis plan is available in the Supplementary Information. Further details regarding research design are provided in the Nature Portfolio Reporting Summary.
References:
33 Jaeger, H. et al.Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV 8, e679-e689 (2021).
2 Venter,W. D. F. et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV 7, e666-e676 (2020).


![Tumor Biomarker Discovery: Advancing Precision Chemotherapy | [Year] Tumor Biomarker Discovery: Advancing Precision Chemotherapy | [Year]](https://i0.wp.com/www.futurity.org/wp/wp-content/uploads/2025/12/cancer-tumors-chemotherapy-1600.jpg?resize=330%2C220&ssl=1)



![Tumor Biomarker Discovery: Advancing Precision Chemotherapy | [Year] Tumor Biomarker Discovery: Advancing Precision Chemotherapy | [Year]](https://i0.wp.com/www.futurity.org/wp/wp-content/uploads/2025/12/cancer-tumors-chemotherapy-1600.jpg?resize=150%2C100&ssl=1)


