Promising New Treatment for Gonorrhea Receives FDA Priority Review
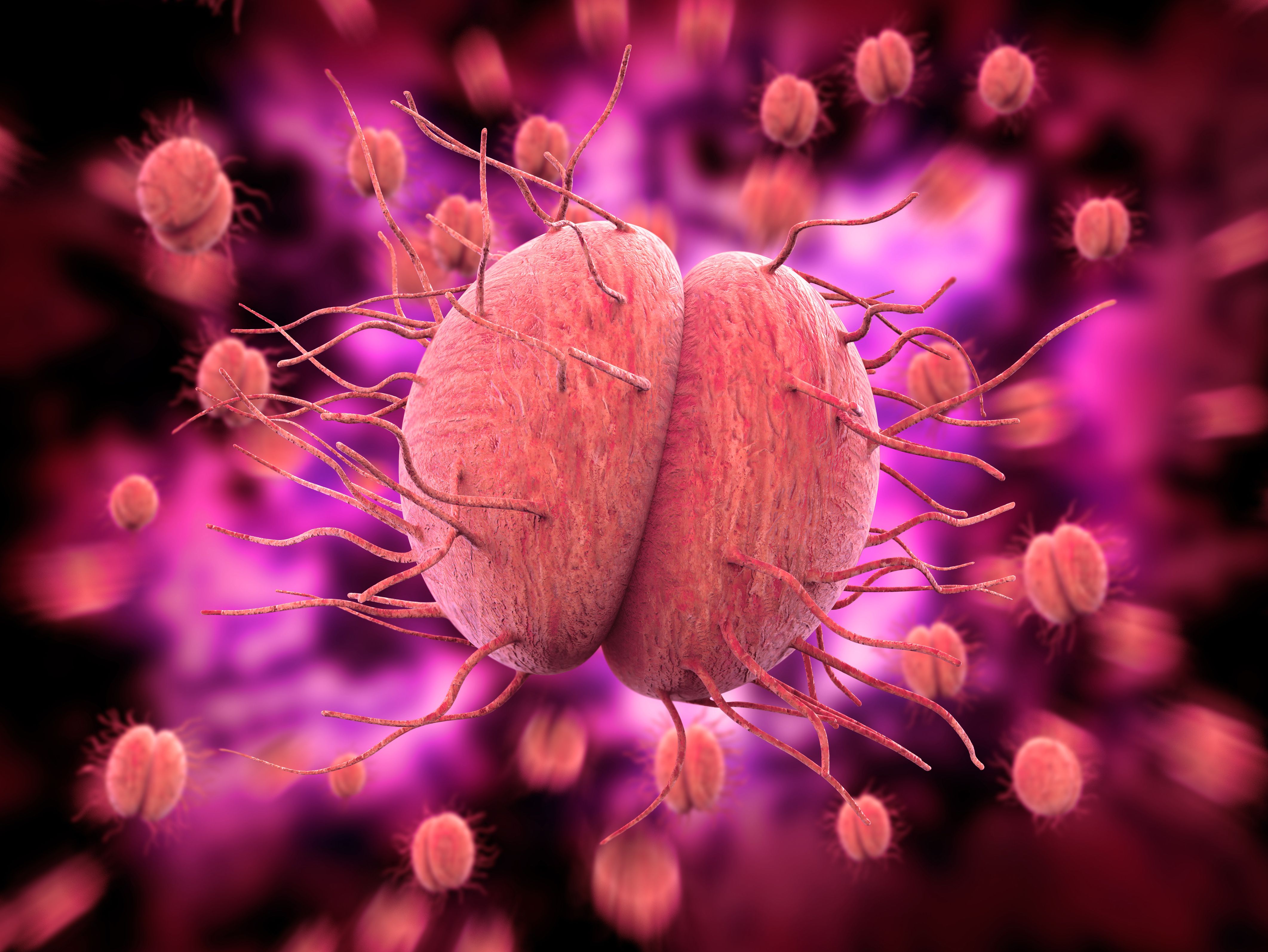
Gonorrhea, a common sexually transmitted infection, is becoming increasingly resistant to existing antibiotics. Fortunately, a potential breakthrough is on the horizon with zoliflodacin, a novel oral antibiotic currently under review by the Food and Drug Management (FDA). This development offers renewed hope for effectively treating uncomplicated gonorrhea infections in adults.
What Makes Zoliflodacin Different?
Zoliflodacin represents a new class of antibiotics, offering a distinct mechanism of action compared to current treatments. This is crucial because gonorrhea’s ability to evolve resistance is a notable public health concern.Existing treatments are losing effectiveness, making new options like zoliflodacin vitally significant.
FDA Priority Review: What Does It Mean for You?
The FDA’s granting of Priority Review signifies the urgent need for new gonorrhea treatments. It means the agency will expedite its review process, aiming for a decision within six months. Typically,standard reviews take considerably longer. This accelerated timeline could mean a new treatment option becomes available to you sooner.
Understanding the Development Process
Here’s a breakdown of key milestones:
* New Drug Submission Acceptance: the FDA officially accepted the application for zoliflodacin,signaling it meets initial requirements for review.
* Priority Review Designation: This highlights the potential of zoliflodacin to address an unmet medical need.
* Ongoing evaluation: The FDA is now thoroughly evaluating the safety and efficacy data submitted by the drug’s developer.
Why is This Important?
Gonorrhea can lead to serious health complications if left untreated, including pelvic inflammatory disease in women and infertility in both men and women. I’ve found that early and effective treatment is key to preventing these outcomes. A new, effective oral antibiotic like zoliflodacin could significantly improve treatment outcomes and help curb the spread of this infection.
What to Expect Moving Forward
While the FDA’s review is underway, it’s important to remember that approval isn’t guaranteed. However, the Priority Review designation is a positive sign. If approved, zoliflodacin would provide a valuable new tool for healthcare providers and offer you a more effective treatment option. Here’s what works best: staying informed about advancements in sexual health and discussing any concerns with your healthcare provider.
It’s a promising development in the fight against antibiotic resistance and a step forward in protecting your health.


![Embryo Implantation Failure: Causes & What You Can Do [Podcast] Embryo Implantation Failure: Causes & What You Can Do [Podcast]](https://i0.wp.com/kevinmd.com/wp-content/uploads/Design-3-scaled.jpg?resize=330%2C220&ssl=1)






![Embryo Implantation Failure: Causes & What You Can Do [Podcast] Embryo Implantation Failure: Causes & What You Can Do [Podcast]](https://i0.wp.com/kevinmd.com/wp-content/uploads/Design-3-scaled.jpg?resize=150%2C100&ssl=1)