Rigorous Statistical Methodology in a Phase 1-4 Clinical Trial of VER-01 for Chronic Neuropathic Pain
This document details the complete statistical methodology employed throughout a four-phase clinical trial investigating VER-01, a novel therapeutic agent for chronic neuropathic pain. The trial, progressing from initial safety assessments (Phase 1) to long-term efficacy and safety evaluation (Phase 4), utilized a variety of advanced statistical techniques to ensure robust and reliable results. This detailed overview underscores our commitment to data integrity and the generation of clinically meaningful insights.
Addressing Missing Data: A Multi-Imputation Strategy
A important challenge in longitudinal clinical trials is handling missing data. To mitigate potential bias, a complex multiple imputation (MI) approach was implemented. Following established best practices52, a two-step MI process was utilized. Initially, a Markov Chain Monte Carlo (MCMC) imputation model generated 100 imputed datasets, transforming potentially complex missing data patterns into a monotone structure. This facilitated the application of a monotone regression model in the second MI step, again generating 100 imputed datasets.Final parameter estimates were then pooled using Rubin’s rule, a statistically sound method for combining results from multiple imputations. As a supportive analysis, Last Observation Carried Forward (LOCF) and Best/Worst observation carried Forward (BOCF) imputation methods were also performed for sensitivity analysis, allowing for comparison and validation of the MI results.This rigorous approach ensures the robustness of findings even in the presence of incomplete data.
Phase-Specific Statistical Analyses
Each phase of the trial employed tailored statistical analyses designed to address its specific objectives:
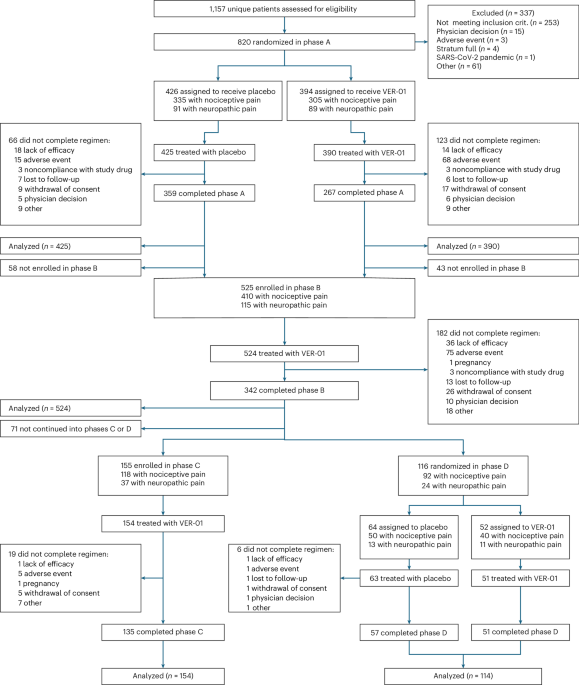
* Phase A (initial Safety & Dose Escalation): The primary endpoint was analyzed using standard statistical methods appropriate for early-phase trials.
* Phase B & C (Efficacy & Long-Term Safety): The key secondary endpoint was evaluated using an Analysis of covariance (ANCOVA) model. This model incorporated treatment as the primary effect and adjusted for key baseline characteristics – NPSI total score, age, sex, and country – to isolate the treatment effect. The same robust MI strategies applied to the primary endpoint were also used here. Safety was assessed through incidence rates of Adverse Events (AEs),with post-hoc comparisons utilizing two-sided chi-squared tests.
* Phase D (Confirmatory Efficacy – Time to Treatment Failure): This phase focused on time-to-event analysis, specifically time to treatment failure. A Cox proportional hazards model was employed, incorporating treatment as the main effect and adjusting for baseline factors including the presence of a neuropathic pain component, NRS pain intensity at week 43, age, and sex. Treatment failure events were clearly defined (e.g., overdosage of rescue medication, study discontinuation due to intolerability, lack of efficacy, or noncompliance). Importantly, data from participants experiencing intercurrent events not indicative of treatment failure were handled using the Missing at Random (MAR) assumption and imputed accordingly. Study discontinuation for reasons unrelated to treatment failure was treated as censored data in the time-to-event analyses.
Sample Size Justification & Power Considerations
Sample size calculations were meticulously performed to ensure adequate statistical power. For Phase D, the pivotal confirmatory phase, a treatment failure rate of 25% for VER-01 and 55% for placebo was assumed, informed by prior research53. Based on this assumption, a sample size of 78 participants (39 per treatment group) was determined to achieve 80% statistical power with a two-sided significance level of 5% when comparing survival curves using the log-rank test. 500 participants were planned for Phase B to collect efficacy and safety data over 6 months, and an additional 150 participants were enrolled in Phase C to gather long-term data for at least 100 participants over 12 months.
Data Integrity & Analysis Procedures
Throughout the trial, rigorous data management and analysis procedures were followed. Analysis sets were clearly defined:
* Full Analysis Sets (FAS): Included all randomized participants who received at least one dose of study medication.
* Safety Analysis Sets (SAS): Included all participants who received at least one dose of study medication,analyzed as treated.
Crucially, an autonomous clinical research organization (CRO) conducted all statistical analyses using SAS software, version 9.4 (SAS Institute). The absence of an independent Data Monitoring Committee (DMC) was a intentional decision based on the trial’s risk profile and was documented accordingly.
**Commit