Rigorous Validation of Dietary Intervention: Sensitivity Analyses and Study Integrity
This research employed a robust methodology,and to ensure the reliability of our findings regarding the impact of ultra-processed (UPF) versus minimally processed food (MPF) diets,we conducted a series of extensive sensitivity analyses. These analyses were designed to assess the robustness of our results to various assumptions and potential biases. This commitment to transparency and thoroughness is central to establishing confidence in our conclusions.
Addressing Potential Data concerns
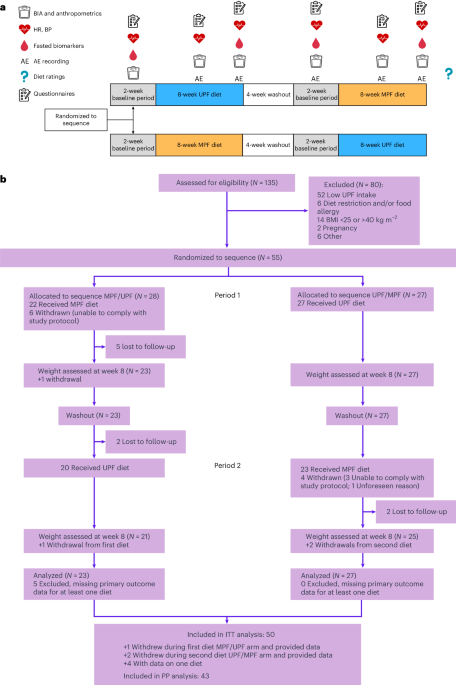
We began by comparing unadjusted analyses of both primary and secondary outcomes at 8 weeks against baseline data.Differences in changes from baseline were evaluated using paired t-tests. Further analyses explored changes at both 4 and 8 weeks,utilizing repeated-measures mixed-effects models to account for the crossover design. We also examined results specifically for the per-protocol (PP) sample, focusing on participants who adhered closely to the study protocol.To further validate our findings, we analyzed data from only the first period of each participantS randomization, providing an independent assessment of the initial dietary effects. Given the nature of a 2×2 crossover design,assessing and adjusting for carryover effects – were the impact of one diet influences the response to the subsequent diet – proved impractical. This limitation is acknowledged, referencing established guidance on crossover trial methodology43. Importantly, no interim analyses were performed, preserving the integrity of the study’s blinding and reducing the risk of bias.
Handling Missing Data wiht Precision
Recognizing that missing data is a common challenge in clinical research, we proactively addressed its potential impact. We employed two sophisticated techniques: multiple imputation with chained equations and inverse probability weighting.
For multiple imputation, we initially imputed missing primary outcome data using variables directly related to the study (diet, randomization arm, nightshift status, and percentage of weight change). We then expanded this model to include a broader range of baseline characteristics – ethnicity, sex, occupation, education, family history of obesity, baseline metabolic rate, energy intake, and weight – to assess the sensitivity of our results to different imputation strategies.
Inverse probability weighting was used to create a reweighted sample, accounting for the probability of receiving each treatment (MPF or UPF diet). Propensity scores were initially calculated based on key stratification variables (sex,ethnicity,nightshift status,and baseline metabolic rate),and then refined by incorporating the expanded set of baseline characteristics.Stabilized weights were then applied to adjust for any imbalances.
Analytical Tools and Ethical Oversight
All statistical analyses were conducted using Rv2024041+748DatapresentationutilizedMicrosoftExcelv1691(24111020)whilevisuallycompellingfiguresweregeneratedwithPrism10v1023AstatisticalimportancethresholdofP < 0.05 was consistently applied. This study was conducted with the highest ethical standards. Approval was granted by the Yorkshire and The Humber-sheffield Research Ethics Committee (22/YH/0281) and prospectively registered on ClinicalTrials.gov (NCT05627570).All participants provided fully informed written consent prior to any study-related procedures. Patient-Centric Design and Transparency
We firmly believe in the importance of patient involvement in research. NHS staff at UCLH provided valuable input during the trial design phase,informed by a dedicated focus group. Furthermore, members of Obesity Empowerment Network UK, individuals with lived experience of obesity, actively contributed to shaping the study’s design. A lay representative served on the trial steering committee, ensuring a patient outlook throughout the research process. Participants were also offered the prospect to consent to a lay summary of the trial results, promoting transparency and accessibility.
For a more detailed overview of our research design, please refer to the Nature Portfolio Reporting Summary.
Key Takeaways:
Rigorous Validation: Multiple sensitivity analyses were performed to confirm the robustness of the study’s findings.
Data Integrity: Sophisticated methods were employed to address missing data and minimize potential bias.
ethical Conduct: The study adhered to the highest ethical standards, with full transparency and patient involvement.
Transparency & Reproducibility: Detailed facts on analytical tools and reporting is readily available.
This meticulous approach